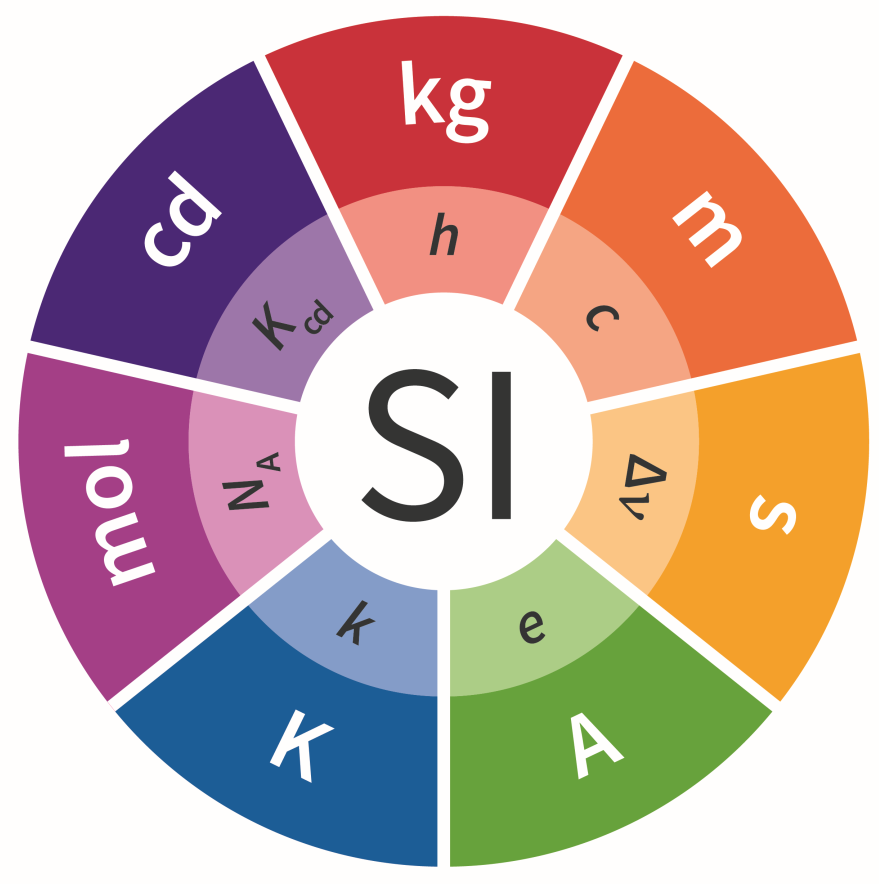
The International System of Units (SI) provides the basis to which we measure the world around us, and ensures that measurements made anywhere in the world are comparable. The SI system is the basis of commerce, allowing goods to be valued and exchanged with confidence and to support every aspect of scientific research.
On November 16, 2018, the unanimous historic vote of the Member States and Associate Members at the 26th General Conference on Weights and Measures (CGPM) approved the upgrading of the International System of Units, that is, the redefinition of the SI system, which dates back to 1960 and represents an international an agreed basis for demonstrating measurements at all levels of accuracy and in all fields of science, technology and human action. The revised SI lists new definitions of four basic units: kilogram, ampere, kelvin and mole, which are based on the determined values of four natural constants: Planck constant (h), elemental charge (s), Boltzmann constant (k) and Avogadro number (NA) respectively. The redefinition of the four basic units at the same time is unprecedented and requires simultaneous global cooperation in different metrology fields.
The definitions of all seven SI units since May 20, 2019, when the new definitions for kg, ampere, kelvin and mole came into force, have been uniquely expressed through the explicit formulations of constants, and specific mises en pratique have been established so to explain the implementation of the definition of each of the basic units in practice. Redefinition of the SI system establishes a set of seven fundamental constants, known as the "defining constants of the SI system":
- The frequency of transition between two non-perturbed hyperfine levels of the ground state of the Cesium atom 133 ΔνCs 9 192 631 770 Hz (hertz),
- The speed of light in vacuum c is 299 792 458 m/s (meter per second),
- Planck's constant h is 6,626 070 15 x 10–34 Js (joule second),
- Elemental charge e is 1,602 176 634 x 10-19 C (coulomb),
- Boltzmann's constant k is 1,380 649 x 10-23 J/K (joule per kelvin),
- Avogadro constant NA is 6,022 140 76 x 1023 /mol (per mole),
- The luminous efficiency of Kcs monochromatic radiation at a frequency of 540 x 1012 Hz is 683 lm/W (lumen per watt).
Definitions of the basic SI units (*)
| Base unit | Definition | Conversion | Diagram |
|---|---|---|---|
| Second (s) | The second is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency ΔνCs, the unperturbed ground-state hyperfine transition frequency of the caesium 133 atom, to be 9 192 631 770, when expressed in the unit Hz, which is equal to s-1. | 1s = 9 192 631 770/ΔνCs | |
| Meter (m) | The metre is the SI unit of length. It is defined by taking the fixed numerical value of the speed of light in vacuum c to be 299 792 458 when expressed in the unit ms-1, where the second is defined in terms of the caesium frequency ΔνCs | 1m = (c/299 792 458)s = 30,663 318... c/ΔνCs | |
| Kilogram (kg) | The kilogram is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h, to be 6,626 070 15 x 10-34 when expressed in the unit Js, which is equal to kgm2s-1, where the metre and the second are defined in terms of c and ΔνCs. | 1kg = (h/6,626 070 15x10-34)m-2s = 1,475 52... x 1040hΔνCs/c2 | |
| Ampere (A) | The ampere is the SI unit of electric current. It is defined by taking the fixed numerical value of the elementary charge e to be 1,602 176 634 x 10-19 when expressed in the unit C, which is equal to As, where the second is defined in terms of ΔνCs. | 1A = (e/1,602 176 634x10-19) x s-1 = 6,789 686 x 108ΔνCs | |
| Kelvin (K) | The kelvin is the SI unit of thermodynamic temperature. It is defined by taking the fixed numerical value of the Boltzmann constant k to be 1,380 649 x 10-23 when expressed in the unit JK-1which is equal to kgm2s-2K-1, where the kilogram, metre and second are defined in terms of h, c and, ΔνCs. | 1K = (1,380 649 x 10-23/k) kgm2s-2 = 2,266 665... ΔνCs h/k | |
| Mole (m) | The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6,022 140 76 x 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol-1 and is called the Avogadro number. The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. | 1mol = 6,022 140 76 x 1023/NA | |
| Candela (cd) | The candela is the SI unit of luminous intensity in a given direction. It is defined by taking the fixed numerical value of the luminous efficacy of monochromatic radiation of frequency 540 x 1012 Hz, Kcd to be 683 when expressed in the unit lm W-1, which is equal to cd sr W-1 or cd sr kg-1 m-2 s3 where the kilogram, metre and second are defined in terms of h, c and ΔνCs. | 1 cd = (Kcd/683) kgm2s-3sr-1 = 2,614 830... x 1010 (ΔνCs)2 h Kcd |
(*) The International System of Units (SI) (9th edition) - https://www.bipm.org/en/publications/si-brochure/
Legal units of measurement in Bosnia and Herzegovina
Legal units of measurement in Bosnia and Herzegovina are established by the SI system of units.
The Law on Units of Measurement ("Official Gazette of BiH", No. 19/01) is in force, and changes are expected with respect to the changes defined for the four basic units of measure kg, A, K and mole from May 20, 2019.
Basic terms and definitions
International Vocabulary of Metrology – Basic and general concepts and associated terms (VIM) JCGM 200:2012 (3rd edition) - https://www.bipm.org/en/publications/guides/vim.html
Measurement unit is a real scalar quantity, defined and adopted by Convention, with which any other quantity of the same kind can be compared to express the ratio of the two quantities as a number.
Measurement standard (etalon) is realization of the definition of a given quantity, with stated quantity value and associated measurement uncertainty, used as a reference.
Metrological traceability (traceability) is property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty.
Calibration is an operation that, under specified conditions, in a first step, establishes a relation between the quantity values with measurement uncertainties provided by measurement standards and corresponding indications with associated measurement uncertainties and, in a second step, uses this information to establish a relation for obtaining a measurement result from an indication.
Prefixes in decanary numeral system
| Symbol | Prefix | Value | Factor |
|---|---|---|---|
| J | jota | 1 000 000 000 000 000 000 000 000 | 1024 |
| Z | zeta | 1 000 000 000 000 000 000 000 | 1021 |
| E | eksa | 1 000 000 000 000 000 000 | 1018 |
| P | peta | 1 000 000 000 000 000 | 1015 |
| T | tera | 1 000 000 000 000 | 1012 |
| G | giga | 1 000 000 000 | 109 |
| M | mega | 1 000 000 | 106 |
| k | kilo | 1 000 | 103 |
| h | hekto | 100 | 102 |
| da | deka | 10 | 101 |
| d | deci | 0.1 | 10-1 |
| c | centi | 0.01 | 10-2 |
| m | mili | 0.001 | 10-3 |
| µ | mikro | 0.000 001 | 10-6 |
| n | nano | 0.000 000 001 | 10-9 |
| p | piko | 0.000 000 000 001 | 10-12 |
| f | femto | 0.000 000 000 000 001 | 10-15 |
| a | ato | 0.000 000 000 000 000 001 | 10-18 |
| z | zepto | 0.000 000 000 000 000 000 001 | 10-21 |
| y | jokto | 0.000 000 000 000 000 000 000 001 | 10-24 |









